46+ calculate the ph of a 2.0 m solution of h2so4
Web The molarity would be the same whether you have 5mathrmmL of ceH2SO4 or a swimming pool full of it. Web What is the pH of this H2SO4 solution.

Calculate The Ph Of A Solution Of 0 0025m H2so4please Help I Dont Get How To Do These Brainly Com
Web From the stoichiometry of above equation it is clear that 1 mole of H₂SO₄ will produce 2 mole of H ions.

. Web H H 2SO4 0325M and work this in to our second proton dissociation calculation Ka2 x0325 x 0325 x 12 102 x 00112 Hence the pH of our. Web The procedure to use the pH calculator is as follows. Second calculate the molarity of the NaOH solution.
Thus each H 2SO 4 molecule gives two H ions. Web Find H concentration and substitute it to pH equation. Web Calculate the pH of a 20-M mathrmH_2 mathrmSO_4 solution.
And second is the concentration of H2SO4. Web Verified by Toppr Correct option is B H 2SO 4 dissociates into 2H and SO 42. First dissociation of is.
Web The first protonation of H2SO4 is assumed to be that of a strong acid meaning there is complete dissociation of the first H. Web The calculator uses the formula M 1 V 1 M 2 V 2 where 1 represents the concentrated conditions ie stock solution molarity and volume and 2 represents the diluted. Step 1 of 5.
Web You can calculate pOH. Then convert to molarity concentration using. Web First convert the mass of NaOH to moles.
Therefore you solution will have at. 100 4 ratings for this solution. Because NaOH is a strong base and is soluble the OH will be equal to the.
Web Consider a sample of a hydrocarbon a compound consisting of only carbon and hydrogen at 0959 atm and 298 K. Chemistry Chapter 14 Acids and Bases Discussion You must be signed in to discuss. Based on equilibrium concentrations of H and OH in water above pH and pOH are related by the following equation which is true for any.
Upon combusting the entire sample in oxygen you collect a. So 002M of H 2SO 4 will give 2002M H ions. Is a diprotic acid.
Your problem is that you only accounted for the first dissociation of ce H2SO4 a. H concentration H 2 SO 4 concentration 2 H concentration 000896 2 H concentration 001792 M pH. Web Now moving onto the calculation there are two key things we need to carry it out.
Web Begin by finding the molar mass of ce H2SO4 in order to find out to how many moles one gram of it is equivalent. Web Calculate the pH of a 20- M H 2 SO 4 solution. First is the pH equation.
So 2 moles of H₂SO₄ will produce 4 mole of H ions and. Step-by-step solution 100 6 ratings for this solution Step 1 of 4 We have solution. Enter the chemical solution name and its concentration value in the respective input field.
PH -log H. Although H 2 SO 4 is a diprotic acid the first acid dissociation constant is very large So it can be assumed. However if you wanted to solve for moles of ceH2SO4 in.
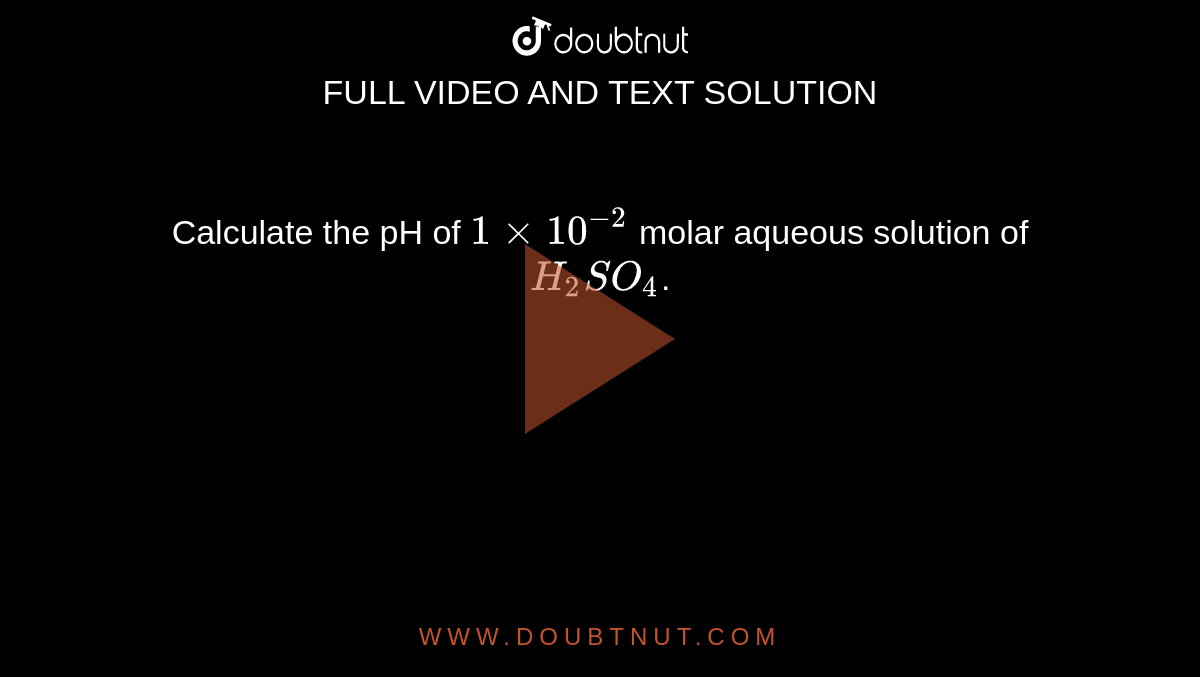
Calculate The Ph Of 1xx10 2 Molar Aqueous Solution Of H2so4

Molarity Calculations
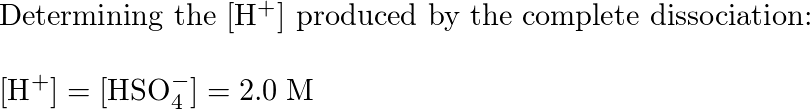
Calculate The Ph Of A 2 0m Solution Of H 2so 4 Quizlet
Calculate The Ph Of 0 05m H2so4 Solution Sarthaks Econnect Largest Online Education Community
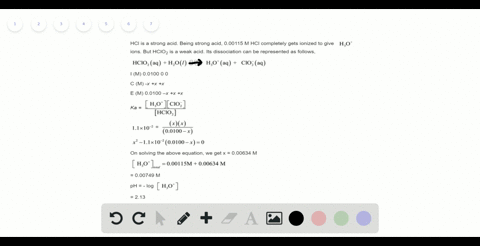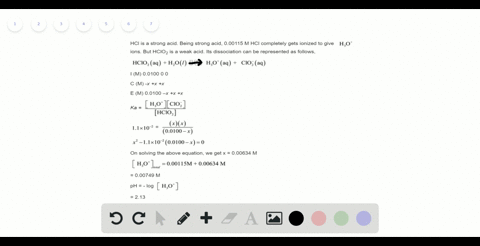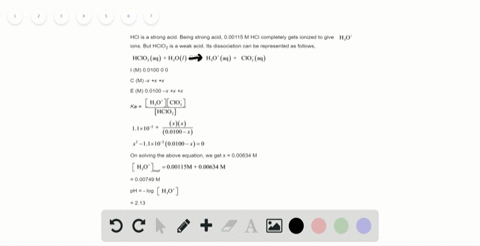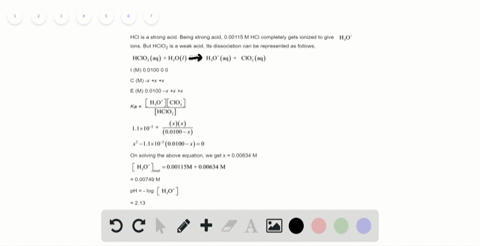
Solved Calculate The Ph Of A 2 0 M H2so4 Solution

Calculate Ph Of A Strong Acid Youtube

The Ph Of 0 005 Molar Aqueous Solution Of Sulphuric Acid Is Approximately

Pubcr Jpg Sample Calculation 2 4 How Many Milliliters Of A 2 0 M Solution Of Boric Acid Must Be Added To 600 Ml Of A Solution Of 10 Mm Sodium Borate Course Hero

View Question What Is The Ph Of A 0 00050m Solution Of H2so4
How Many Milliliters Of 2 00 M H2so4 Are Needed To Provide 0 250 Mole Of H2so4 Quora

Ph Of Sulfuric Acid Youtube
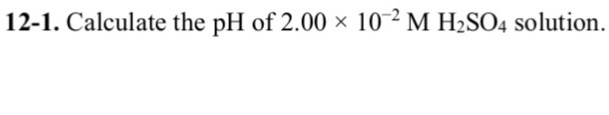
Solved 12 1 Calculate The Ph Of 2 00 10 2 M H2so4 Chegg Com

Caculate The Ph Of 0 001 N H 2 So 4 Solution
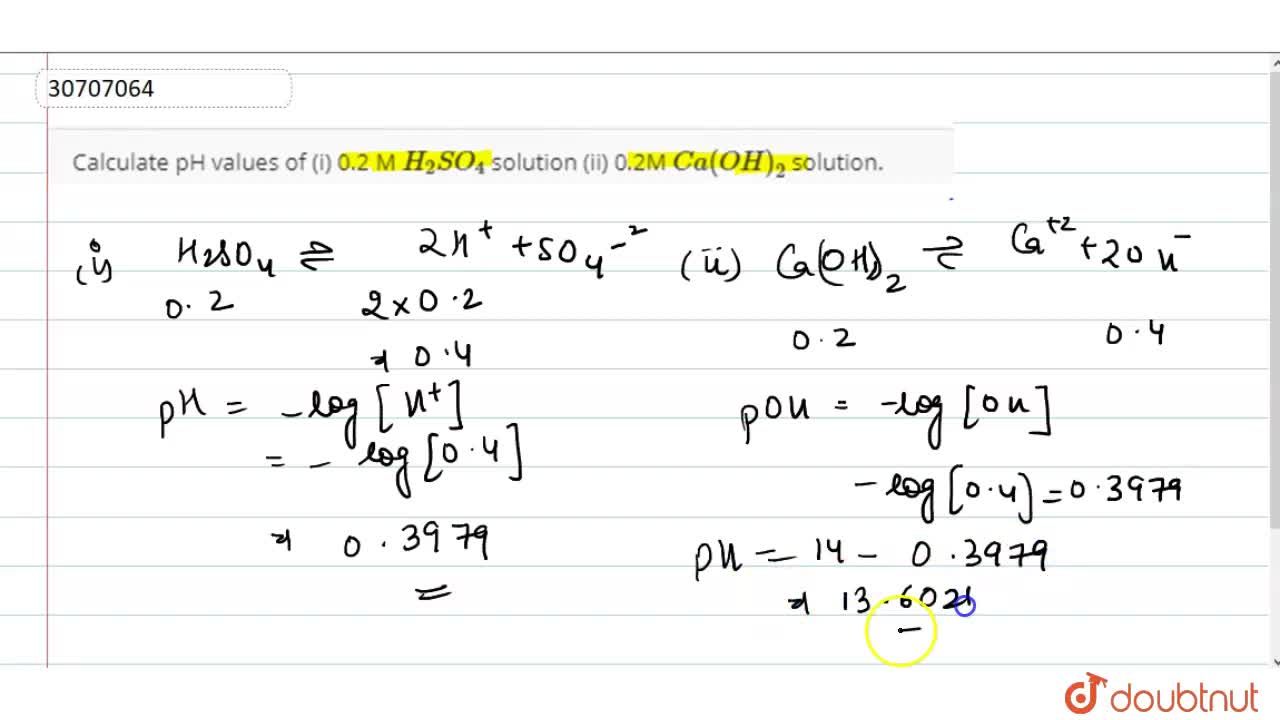
Calculate Ph Values Of I 0 2 M H 2 So 4 Solution Ii 0 2m Ca Oh 2 Solution

Assuming Complete Dissociation Calculate The Ph Of The Following Solutions Youtube
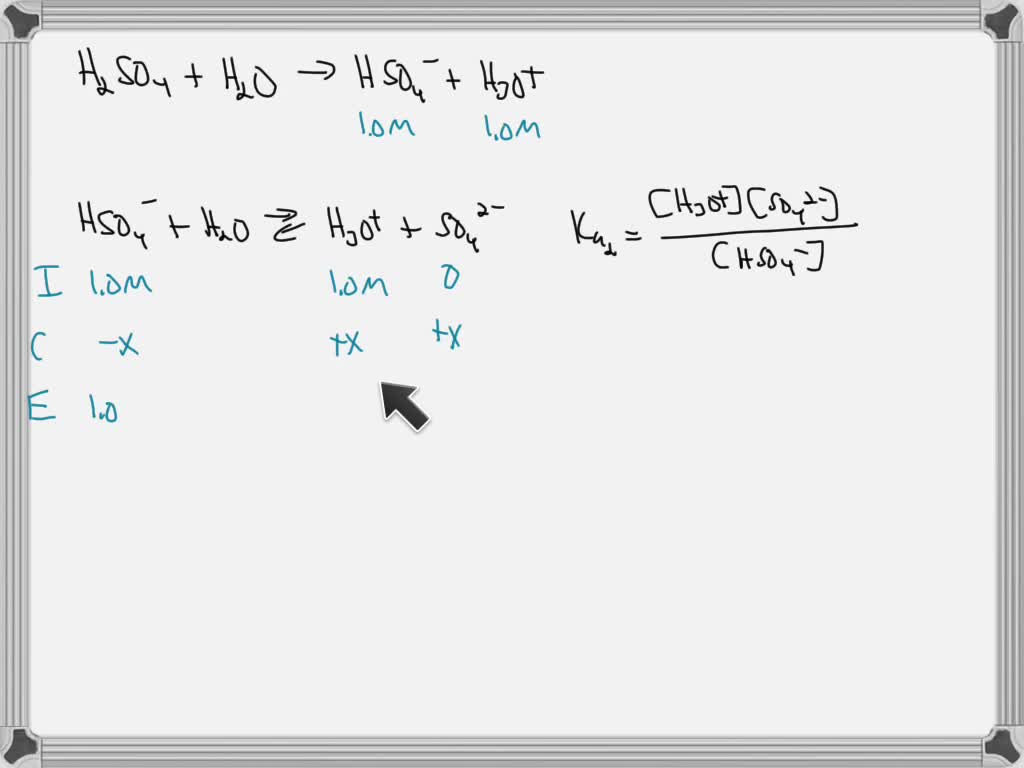
Solved Calculate The Ph And Concentration Of All Species H2so4 Hso4 H3o And So42 In A 0 100m Solution Of Sulfuric Acid Ka1 Strong And Ka2 1 1 X 10 2
How To Calculate The Ph Of 0 1 M H2so4 Quora